
BHK-21 cells remain one of the most reliable and scalable mammalian cell lines in global vaccine manufacturing. Originally derived from the kidneys of juvenile hamsters, BHK-21 cells have developed a strong reputation for robust growth, adaptability to suspension culture, and high virus productivity. Today, they are widely used across veterinary vaccine industries, particularly for foot-and-mouth disease (FMD), pseudorabies, Newcastle disease, bovine epidemic fever, and several other economically significant livestock viruses.
As vaccine manufacturers move toward higher efficiency, lower cost per dose, and more consistent production performance, the demand for specialised BHK-21 culture media continues to rise. This is where the BHK media series, designed specifically for BHK-21 cells, becomes essential—offering scalable, serum-free, animal-component-free solutions optimised for both upstream growth and downstream virus production.
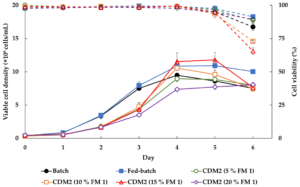
Compared with other mammalian lines, BHK-21 cells adapt exceptionally well to high-density suspension culture. This enables vaccine producers to scale up viral production using bioreactors rather than traditional adherent systems, significantly reducing space, labour and scaling costs.
Typical suspension density:
BHK-21 is one of the preferred host cell lines for:
Producers consistently report:
BHK-21 cells have decades of documented safety, making them widely accepted by global regulatory bodies. This helps minimise approval delays and reduces the burden of validation during commercial manufacturing.
To maximise the potential of BHK-21 cells, their culture environment must provide balanced nutrients, stable osmolality, and minimal variability. The BHK series has been formulated specifically for these needs—optimising cell growth, enhancing virus output, and supporting seamless scale-up.
Below is a breakdown of each product and how it supports the vaccine manufacturing process.
Best for: Cell expansion and virus production during early-stage culture
Low serum media helps reduce production costs while maintaining consistent performance.
Benefits:
This medium is ideal for facilities gradually reducing serum levels without compromising process performance.
Best for: Large-scale, regulatory-compliant suspension culture
Serum-free media are now preferred across advanced vaccine manufacturers due to cost, consistency and biosafety requirements.
Key features:
Facilities switching from serum-based to animal-component-free processes often report significantly improved batch-to-batch reliability.
Best for: Ultra-high-density cultures and industrial bioreactor production
This advanced formulation is engineered for peak cell viability and optimal metabolic performance in long-duration runs.
Advantages:
This is ideal for facilities targeting process intensification or high-throughput manufacturing.
Best for: Virus amplification after cell infection
Once viral inoculation has occurred, the focus shifts from rapid cell growth to creating the optimal metabolic environment for viral replication.
Key functions:
Using a dedicated maintenance medium provides better control over the infection phase—leading to more predictable virus yields.
A powerful way to boost productivity, especially during high-density fermentations.
Feed F and Feed V offer:
These 10000X feeds integrate seamlessly into existing production processes and are suitable for both serum-free and low-serum systems.
Serum-free and low-serum formulations significantly reduce raw material costs and quality risks.
Dry-powder formats are ideal for large-volume downstream preparation and long-term storage.
Animal-component-free media simplify global compliance for GMP facilities.
Combined growth/maintenance systems and targeted feeding strategies help maximise viral titers while maintaining process stability.
BHK-21 cells remain a foundational platform for veterinary virus vaccine production, and the specialised BHK media series ensures that manufacturers can achieve peak performance—from rapid cell expansion to final virus harvest. With low-serum options, fully serum-free formulations, dedicated post-infection maintenance media, and high-efficiency virus production enhancers, the BHK series provides a complete, scalable, and compliant solution for modern bioprocessing.
For Further Enquiry Contact- sales@copure.com.au